Like quality controls built into an assembly line, the body has a vital protein control mechanism that maintains homeostasis. Because proteins play many critical roles in the body, doing most of the work in our cells, this system, called proteostasis, continually tests the quality of the proteins, fixing or sweeping away damaged ones.
Technion researchers have demonstrated, for the first time, how this quality control system breaks down with aging, leading to neurodegenerative diseases of the elderly such as Alzheimer’s and Parkinson’s, as well as Huntington’s and ALS.
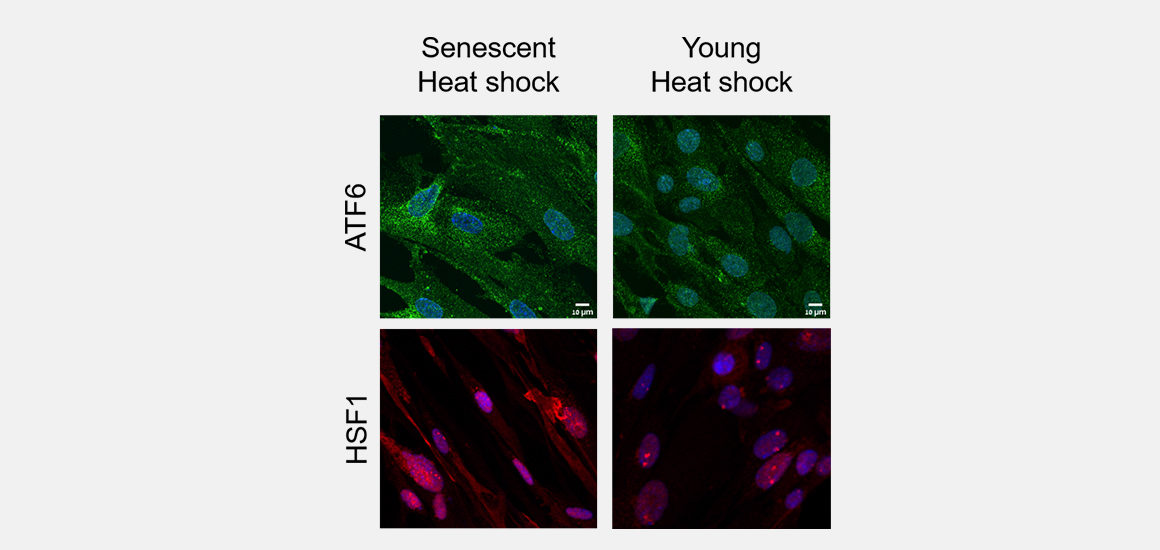
When the body is functioning correctly, proteins undergo a physical process called folding, where they acquire a specific three-dimensional structure that allows them to interact normally with other cell components. Defects in the folding process cause these damaged proteins to become “sticky” and accumulate in cells. This unchecked accumulation has been linked to neurodegenerative diseases.
Professor Reut Shalgi
In a study published in the Proceedings of the National Academy of Sciences, Technion Professor Reut Shalgi and her team in the Rappaport Faculty of Medicine subjected both young and aged proteins to heat to observe how they behave under stress. They found that unlike young cells, the aging cells were unable to trigger two particular responses needed to overcome the stress. Even given time, the aging cells could not recover from the heat shock.
The findings shed light on the forces behind aging, which hold potential for developing treatments to slow the progression of neurodegenerative diseases.
Prof. Shalgi joined the Technion in 2014, where she investigates questions related to protein homeostasis regulation in stress and neurodegeneration. She is a member of the Prince Center for Neurodegenerative Disorders of the Brain.